In manufacturing and distribution companies, conformity in quality is vital for a sustainable business. If conformity is not managed correctly, it can affect the efficiency and effectiveness of the quality management system. When deviations or non-conformities occur, immediate action is required to avoid issues that can ruin a company’s reputation and disrupt day-to-day operations and be very costly.
The International Organization for Standardization (ISO), a globally recognized authority, defines the requirements for controlling and documenting non-conformances in quality management. It’s essential to ensure that products meet compliance requirements and standards such as ISO 9001:2015. This standard outlines how organizations can achieve an effective quality management system, leading to enhanced customer satisfaction.
In this blog post, you’ll learn about non-conformances in quality management and how to deal with them.
What is Non-Conformance in Quality Management?
Non-conformance (NC), also known as non-conformities, occurs when a product, process, or service doesn’t meet the specified requirements. Deviations can occur for many reasons, such as quality defects due to tear and dent, contamination, technical problems, and human error. Dedicated personnel in the organization must address these deviations and take corrective actions promptly.
It’s essential to document non-conformances in quality management to ensure continuous improvement in the process. When you address the issue at an early stage, it saves you from bigger problems such as hefty rework, product recalls, production delays, and low productivity, thereby promoting a proactive approach to quality management.
Types of Non-Conformances
Non-conformances or non-conformities are commonly categorized into two types: major and minor. The key difference lies in the level of its impact. The non-conformance level can differ from one industry to another.
Major – Major non-conformances seriously affect the quality of products and services, as well as safety, health, and the overall outcomes of the product usage. Major non-conformances require immediate corrective action to prevent hazardous results, which can lead to significant consequences for a company. Examples of major non-conformances include products that do not meet quality standards requirements such as ISO 9001, not following SOPs, untested products, lack of training, and more.
Minor – Minor non-conformances, on the other hand, do not have a major impact on the production, process, or quality of products or services. They can be easily managed and addressed, and they do not have a major affect on the safety and health of consumers. Examples of minor non-conformances include missing documentation, improper calibration of equipment, invoice mistakes, and more.
How To Deal with Non-Conformances and Deviations in Quality Management?
Dealing with non-conformance is a systematic approach of identifying, documenting, investigating, and resolving the product, process, and service to ensure a concrete action plan is implemented to prevent the recurrence of NC. Manufacturing and distribution companies can follow this process to handle quality non-conformance and deviations.
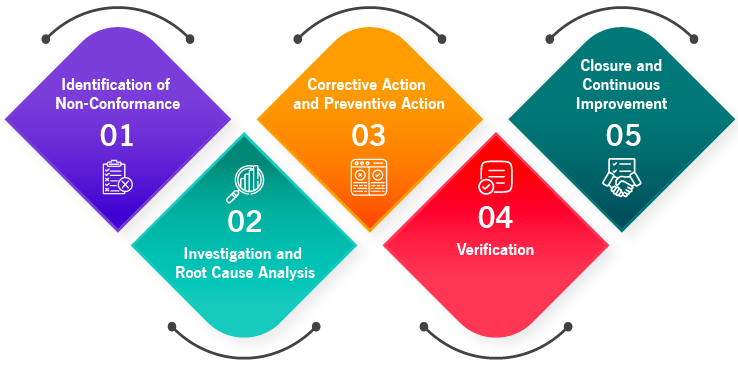
1. Identification And Documentation of The Non-Conformance
Non-conformance can occur due to internal or external factors. Internal factors may include not following standard operating procedures (SOPs), deficiencies found during internal audits, and incorrect testing procedures. External factors may encompass customer complaints, findings from external audits, and issues with suppliers. Whenever encountering a process, product, or service failure, it is crucial to promptly identify and document the deviation. Providing thorough details of the deviation enables precise analysis of the situation. In case of major deviations and non-conformance, the quality control department needs to be informed and take needed corrective actions and resolve the issue in a timely manner. Subsequently, appropriate containment measures could be taken, which may involve segregating defective products, isolating them, or temporarily halting shipments.
2. Investigation and Root Cause Analysis (RCA)
After identifying and documenting non-conformance, it’s crucial to analyze the root cause of the issue. Investigate the actual cause and its origin to understand why the deviation occurred. Respond to the problems in a systematic way. Assign personnel or departments to conduct this analysis, utilizing methods such as pareto chart, fishbone diagram, 5W1H, and 5Whys technique.
3. Documentation of Corrective and Preventive Action (CAPA)
After thoroughly investigating and knowing the actual cause and its origin, it is essential to implement corrective and preventive actions. Corrective actions address and eliminate the identified issue, rectify errors that occurred. Preventive actions are aim at preventing the recurrence of an issue in the future. For example, reworking the defective product is a corrective action, whereas improving material quality, updating processes, and providing shop floor training are preventive actions. Corrective actions are reactive, and preventive actions are proactive.
Develop a plan to implement both corrective and preventive actions by adopting approaches like PDCA (Plan-Do-Check-Act) to plan and implement CAPA. More than one corrective and preventive action can be planned and documented. Once the actions are planned, communicate and notify the team or personnel to enhance the effectiveness of the quality management system. This ensures thorough tracking and monitoring of all implemented actions.
4. Verification
After implementing corrective actions, documenting CAPA details, and notifying personnel, it is crucial to verify the process. The verification includes ensuring the CAPA is implemented correctly, and non-conformances are addressed satisfactorily. This stage assesses the effectiveness of the CAPA and whether the non-conformances are resolved effectively with no discrepancies found.
5. Closure and Continuous Improvement
Finally, it’s time to close the non-conformance. Implement the system to track and monitor the documented details of NC and CAPA in a centralized location. Regularly monitor and review the process to promote continuous improvement in the organization. It’s essential to keep a record and track of the non-conformance to effectively prevent non-conformance in the future. This enhances greater transparency across departments and can be helpful during audits.
Benefits of Non-Conformance (NC) Management
Gain tremendous benefits by managing non-conformances with a well-structured procedure to meet the specific needs and requirements of your organization. Understanding the patterns of the issues, identifying non-conformances, and implementing effective corrective and preventive actions can help the organization in many ways:
- Facilitates continuous improvement.
- Ensures consistent delivery of quality products and services.
- Enabling organizations to quickly identify and track the issues as they arise and work on them to avoid potential consequences.
- Enhance customer satisfaction by ensuring products meet specifications.
- Help avoid fines and penalties associated with non-compliance.
- Address quality gaps, leading to continuous improvement of the processes, products, and services.
Wrap Up
Non-conformance (NC) or deviation management is a crucial part of a comprehensive quality management system. Companies that aim to effectively manage the deviations in quality with a systematic approach can reduce the impact and negative consequences of the non-conformances in the organization.
Documentation of NC and CAPA with the right system helps to keep track of the records and prevents them from being overlooked. A comprehensive quality management system with robust NC CAPA management, like eWorkplace Apps’ Quality Management Suite for Acumatica enables manufacturers and distributors to maintain compliance and manage deviations in the quality of the product, process, and services. Digitize and streamline the process in the organization with the right system to speed up the process in today’s fast-paced environment.
Curious about how you can effectively handle non-conformances with the right solution? Contact us to learn more.
Contact Us
"*" indicates required fields